A publication in Nature reports the successful regeneration of ~80% of the skin of a patient with junctional epidermolysis bullosa (JEB) using a combination of ex vivo gene therapy and stem-cell replacement therapy. The procedure represents a major stepping stone in the translation of stem cell-based therapies to the clinic.
“this study paves the way for other stem cell-based therapies”
JEB is a severe genetic disease caused by mutations in three genes (LAMA3, LAMB3 and LAMC2) that together encode the heterotrimeric basement membrane component laminin, as well as mutations in genes that encode collagen and integrins. These mutations cause defects in the anchoring of the epidermis to the underlying dermis and, as a result, the skin becomes very fragile. Minor traumas cause epidermal detachment and fragmentation, resulting in large, chronic wounds. Patients are at high risk of infection or skin cancer. JEB is usually lethal early in life and has no cure.
The patient was a 7-year-old child carrying a homozygous mutation in the LAMB3 gene, which had led to complete epidermal loss on about 60% of his total body surface area (TBSA). The treatment restored healthy epidermis in about 80% of the child’s TBSA.
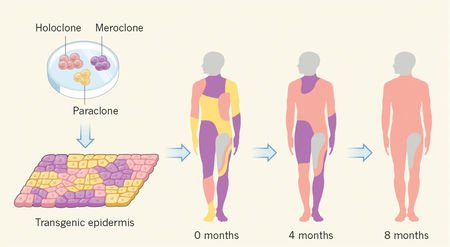
The authors established primary keratinocyte cultures from a biopsy sample taken from a non-blistering skin area. The cells were transduced with a retroviral vector expressing full-length LAMB3 cDNA and were cultivated on either plastic or fibrin substrates to produce transgenic epidermal sheets. These sheets were applied to the exposed dermis and showed full engraftment in most of the transplanted areas after 10 days. Epidermal regeneration was complete after 1 month, restoring ~0.85 m2of the child’s skin. Now, about 2 years after the beginning of the treatments, the epidermis of the child is stable and robust.
Biopsy samples taken from random locations at 4, 8 and 21 months after grafting showed that the regenerated epidermis consisted only of transgenic keratinocytes, which produced normal amounts of laminin and in which cell adhesion and a functional basal lamina were restored.
Using high-throughput sequencing, the authors investigated the genomic integration profile of the retroviral vector in pre- and post-graft samples. They found that the majority of integrations occurred in non-coding regions (~10% in promoters, <5% in exons, ~47% in introns and ~38% in intergenic regions); the genes with integrations are not known to be associated with cancer. Importantly, there was no significant difference in the distribution of insertions between pre- and post-graft samples, indicating that the integration pattern is maintained in vivo, and that epidermal renewal was not associated with the proliferation of selected clones (which could lead to tumours).
The study also provides key insights into the mechanisms of skin regeneration. As a result of very high cell turnover, the entire human epidermis is replaced each month. However, it was not clear whether long-term epidermal renewal is sustained by a small population of long-lived stem cells or by a large population of equipotent keratinocyte progenitors that derive from the stem cells. Sequencing of the biopsy samples taken 8 months after grafting revealed a relatively small number of different genotypes (~200–400) compared with the high number of vector integration sites found in culture (~27,000), indicating that a few long-lived transplanted stem cells sustained the renewal of human epidermis.
Long-term follow-up of patients will be important to assess the safety of the described treatment. Nevertheless, this study paves the way for other stem cell-based therapies.
[“Source-nature”]