
Bertrand Routy earned a lamentable reputation with Parisian oncologists in 2015. A doctoral student at the nearby Gustave Roussy cancer centre, Routy had to go from hospital to hospital collecting stool samples from people who had undergone cancer treatments. The doctors were merciless. “They made fun of me,” Routy says. “My nickname was Mr Caca.”
But the taunting stopped after Routy and his colleagues published evidence that certain gut bacteria seem to boost people’s response to treatment1. Now, those physicians are eager to analyse faecal samples from their patients in the hope of predicting who is likely to respond to anticancer drugs. “It was an eye-opener for a lot of people who couldn’t see the clinical relevance of gut microbes,” says Routy, who is now at the University of Montreal Health Centre in Canada.
Cancer has been a late bloomer in the microbiome revolution that has surged through biomedicine. Over the past few decades, scientists have linked the gut’s composition of microbes to dozens of seemingly unrelated conditions — from depression to obesity. Cancer has some provocative connections as well: inflammation is a contributing factor to some tumours and a few types of cancer have infectious origins. But with the explosive growth of a new class of drug — cancer immunotherapies — scientists have been taking a closer look at how the gut microbiome might interact with treatment and how these interactions might be harnessed.
After preliminary findings in mice and humans revealed that gut bacteria can sway responses to such drugs, scientists started trying to decipher the mechanisms involved. And researchers are launching a handful of clinical trials that will test whether the gut microbiome can be manipulated to improve outcomes.
Some proponents say that strategies to mould the microbiome could be game-changing in cancer treatment. “It’s a smart place to be,” says Jennifer Wargo, a surgeon–scientist at MD Anderson Cancer Center in Houston, Texas. But others are worried that the move to the clinic is premature. William Hanage, an epidemiologist at the Harvard T. H. Chan School of Public Health in Boston, Massachusetts, calls the idea “phenomenally interesting”, but adds: “I have some anxiety about the notion that only beneficial effects are possible.”
Intriguing link
Although the excitement about microbes and immunotherapy has emerged only in the past three years, some researchers have been exploring connections between gut bacteria and cancer for much longer. Scientists first linked the infectious bacterium Helicobacter pylorito gastric cancer back in the 1990s, for example. And since then, other bacteria have been associated with cancer initiation and progression. Some of these microbes activate inflammatory responses and disrupt the mucus layers that protect the body from outside invaders, creating an environment that supports tumour growth. In other cases, they promote cancer survival by making cells resistant to anticancer drugs.
But gut bacteria can also help fight tumours. In 2013, a group led by Laurence Zitvogel2 at Gustave Roussy and one led by immunologists Romina Goldszmid and Giorgio Trinchieri3 at the National Cancer Institute in Bethesda, Maryland, showed that some cancer treatments rely on the gut microbiome activating the immune system.
Zitvogel’s team found that the chemotherapy drug cyclophosphamide damages the mucus layer that lines the intestine, allowing some gut bacteria to travel into the lymph nodes and spleen, where they activate specific immune cells. For mice raised without microbes in their guts or given antibiotics, the drug largely lost its anticancer effects.
Following this observation, Zitvogel decided to explore whether bacteria in the gut might influence responses to a class of immunotherapy drugs called checkpoint inhibitors. These drugs, typically antibodies to cell-surface molecules such as CTLA4 and PD1, unleash a person’s immune system against tumour cells, and are used to treat several types of cancer (see ‘A little help from their friends’). But only 20–40% of people respond to treatment4.
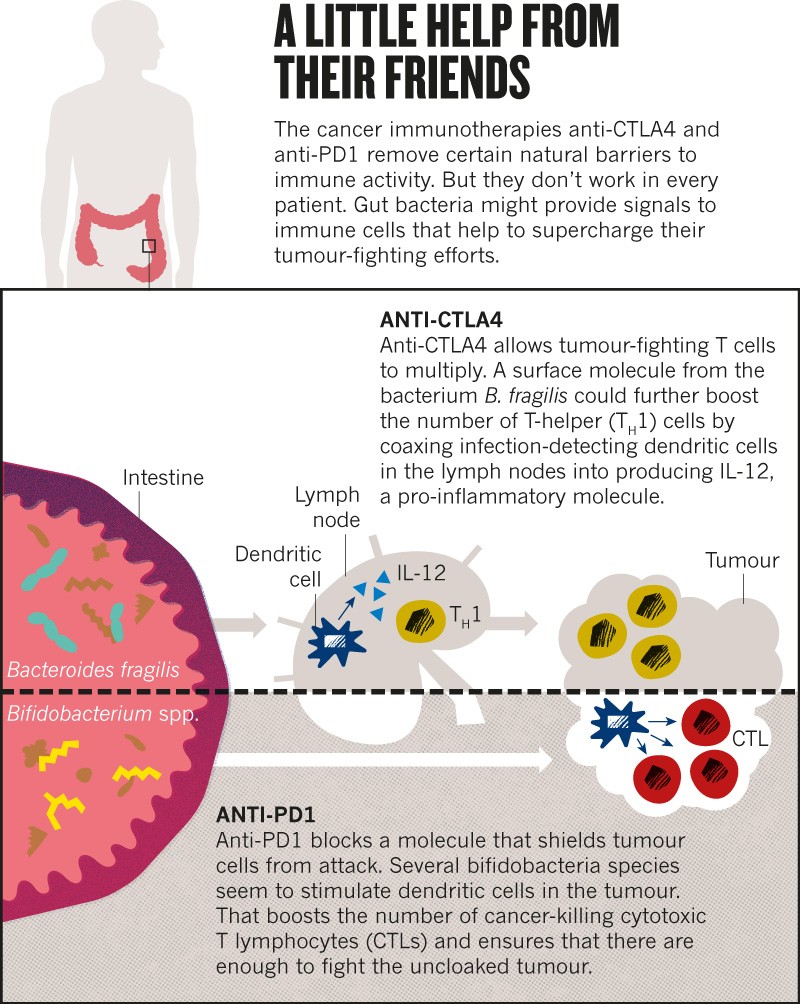
In 2015, Zitvogel and her team showed that microbe-free mice failed to respond to one such drug, and mice given a particular bacterium, Bacteroides fragilis, responded better than did mice without it5.
The idea started to spread. Thomas Gajewski, a cancer clinician at the University of Chicago in Illinois, reported that Bifidobacterium microbes increased the response to cancer immunotherapy in mice6. These gut-dwelling bacteria acted by boosting the ability of some immune cells to initiate a response against tumours.
Wargo saw these results presented at a meeting in 2014, and on returning to Texas, immediately started to collect stool samples from people with skin cancer who were about to undergo immunotherapy at her institution. Last November, Wargo7, Gajewski8 and Zitvogel1 all published results in Science linking positive immunotherapy responses in people to specific varieties of gut bacteria. The samples that Routy had collected in Paris helped Zitvogel’s team to also show that people who had taken antibiotics for unrelated infections tended to respond poorly to immunotherapy.
To solidify the relationships, the researchers transferred bacteria from the human participants into the intestines of mice with comparable cancers. Rodents who got ‘beneficial’ bacteria developed smaller tumours than did mice that received microbes from people who hadn’t responded to treatment. “All of this work has been very exciting,” says Neeraj Surana, a microbiologist at Boston Children’s Hospital. “They’ve opened up the possibility for a clear therapeutic application of microbiome science.”
Heading to the clinic
Researchers are now running with that possibility. Hassane Zarour, an immunologist at the University of Pittsburgh in Pennsylvania, partnered with the global pharmaceutical company Merck to collect faecal bacteria from people who respond to treatment with a checkpoint inhibitor and transfer them into the intestine of non-responders, a process called faecal microbiome transplant. Merck has invested about US$900,000 into this trial, which is set to start in the next few weeks.
Wargo is planning a similar trial. Together with the Parker Institute for Cancer Immunotherapy in San Francisco, California, and the biotech company Seres Therapeutics in Cambridge, Massachusetts, she expects to test whether faecal transplants can reshape the gut microbiome of non-responders in a beneficial way.
These microbiome transplants are becoming a mainstream treatment for some non-cancer illnesses. In February, for example, the Infectious Diseases Society of America recommended that physicians use these procedures to treat people with bowel infections caused by the bacterium Clostridium difficile who had failed to respond to other treatments. But the approach has downsides. To avoid the risk of inadvertently infecting people with pathogenic microbes, researchers must be careful in how they select donors and screen faecal material before transferring it to recipients. That’s why, in addition to faecal transplants, Seres Therapeutics, the Parker Institute and Wargo will test a pill containing a set of spore-forming bacteria that have been purified from the faeces of responding patients.
Gajewski and his partners at Evelo Biosciences, a biotech company in Cambridge, are using a similar approach. Their trial will assess the effects of two pills containing single bacterial strains in people with different types of cancer, including colon and skin cancer.
Zitvogel is not planning to start clinical trials but she has co-founded the Delaware-based start-up EverImmune, which is developing a microbiome-based pill.
It’s still unclear exactly how microbes might interact with immunotherapeutics. A widely accepted hypothesis is that some boost the body’s response against tumours by regulating how easy it is to activate the immune system. But the precise mechanisms, including which bacteria modulate which immune cells, remain a mystery.
The researchers hope that the clinical trials will help to clarify things. Wargo, for instance, is exploring bacterial metabolites. Her team hopes to find specific metabolic signatures of a good outcome in the stools and blood of people who respond to therapy, as well as to document the numbers of immune cells in the blood and tumours of trial participants.
Gajewski suggests that microbes might be unleashing the immune response by stimulating the gut cells to produce certain molecules. His team is testing whether circulating immune-cell precursors change their behaviour when specific bacteria are given to mice. At the same time, the group is trying to pin down which species might be driving the positive outcomes.
Too early, or just right?
Given the uncertainties, some scientists argue that testing these approaches in humans is risky. Some trial participants could experience side effects, Surana says. And changing the make-up of an individual’s microbiome might predispose them to other health problems.
Faecal transplants come with a lot of unknowns. They have proved safe and effective in many people without cancer, Wargo says, but they have also been associated with unexpected effects, including one case in which the procedure led to weight gain and obesity9. “Should we look for safety signals on these trials? Absolutely.” Wargo says, “But I strongly feel that we need to go into these trials. We need to design them well. We need to really learn from these trials.”
Gajewski, who plans to test the effects of just one bifidobacterial strain at a time, says there’s good reason to be confident. “People have eaten bifidobacteria for a thousand years,” he says. The bacteria are present in the gut of infants and decline in number as the people grow up, so they should at least be safe, he adds.
But it’s unclear whether a single species can help people with cancer and, if so, what bacterium that is. The papers published in Science last year all associated different bacteria with the best outcomes, even for the same cancer and therapy.
The researchers looked at people with cancer from France and the United States, so diet could account for some of the differences, Wargo says. But variations in sample collection, data analysis and statistical methods could also have skewed the results, says Joël Doré, a biologist at the French National Institute for Agricultural Research in Paris who in 2011 helped to launch the International Human Microbiome Standards (IHMS) project with the aim of improving data reproducibility in microbiome research.
Hanage says that even the two studies7,8 that analysed people in the United States with the same type of cancer identified only a partially overlapping set of microbes associated with positive outcomes. If researchers don’t work out the reason for these differences, they might not be able to interpret the outcomes of the trials, Hanage says.
Before starting clinical trials, the three groups should try to reproduce each other’s results and converge on a set of ‘beneficial’ microorganisms, Hanage argues. “Any of these bacteria could be a useful approach.” But inconsistencies might mean that the results are not reproducible.
It’s a concern common to microbiome research. “A lot of findings have proven to either not stand up or be considerably more complicated than they first appeared,” Hanage says. Standards such as those developed by the IHMS project should help, but scientists will be reluctant to take them on board, says Susan Erdman, a microbiologist and cancer biologist at the Massachusetts Institute of Technology in Cambridge. Doing so would come at the cost of innovation, she argues — it’s by experimenting in different settings that researchers make discoveries.
Wargo says that the community should standardize its approaches for collecting samples and doing analyses, as well as for validating studies in larger groups of patients. Since last year, her group has analysed stools from more than 500 people with skin cancer who had received different therapies. In parallel with the Paris-based team led by Zitvogel, the researchers are analysing patients treated with two combined immunotherapies to work out which gut bacteria mediate a response to that combination. Wargo hopes that the gut microbiome could eventually help to identify which patients will respond to which anticancer treatments. “Can we use it as a biomarker? It’s a provocative question,” she says.
In the short term, there will be a whole lot more sample collection. And this time around, it’s likely that fewer oncologists will raise an eyebrow, says Routy, who is now investigating how the gut microbiome boosts immunotherapy with his own group. In cancer therapy, “gut microbes have gone from ignored to super-popular organisms”, he says. Now, they’ll just have to live up to their reputation.
source:-nature.
